

There was a strong family history of male deaths in infancy on the maternal side. Past history revealed an episode of cervical lymphadenitis treated with antibiotics at the age of 2 weeks and poor weight gain from 3 months. Here we report the outcome.Ī 6-month old male infant, the first child of non-consanguineous parents, was referred to The Children’s Hospital at Westmead with persistent pneumonia not responding to antibiotic therapy.
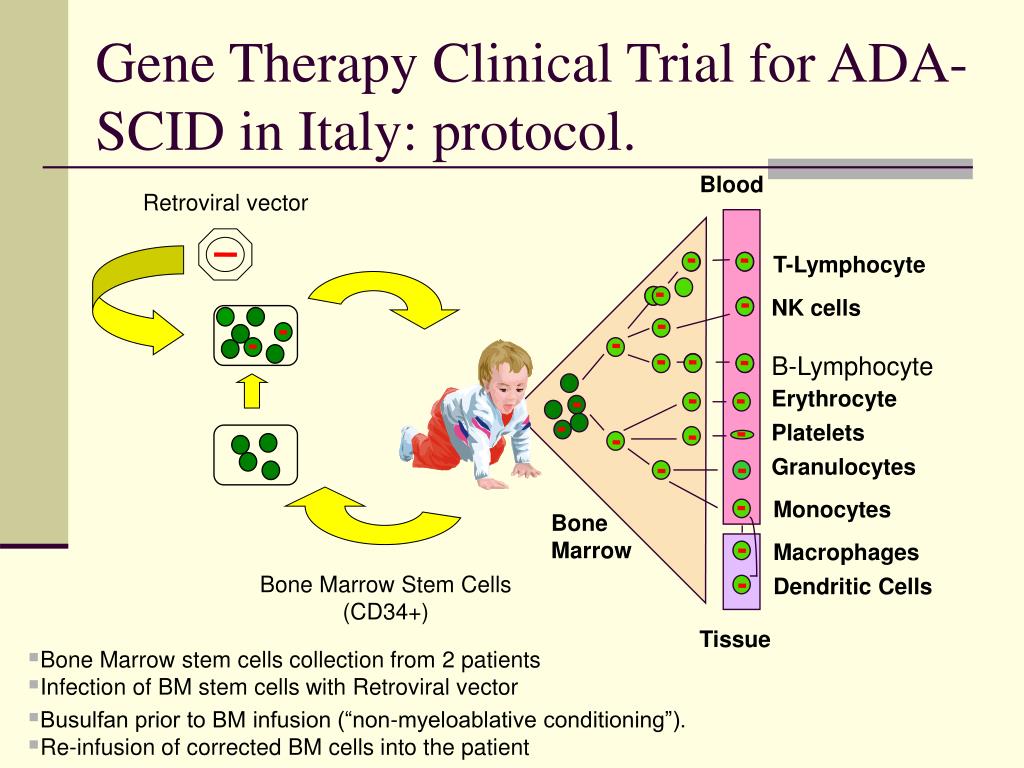
In collaboration with the French team, we treated an infant with SCID-X1 by gene therapy at The Children’s Hospital at Westmead, Sydney, NSW, in March 2002. Gene therapy offers these infants the potential for improved survival rates and more complete immunological reconstitution. 6, 7 In most infants, immunological reconstitution remains incomplete, particularly B-cell function, with resultant lifelong requirement for immunoglobulin replacement therapy. However, most infants lack such a donor and conventionally undergo an HLA-mismatched transplant with associated mortality rates of up to 30%. The treatment of choice for SCID-X1, with greater than 90% survival, is bone marrow transplantation from an HLA-identical sibling donor. 2, 3 Affected infants typically lack both T and natural killer (NK) cells, and have normal or raised levels of functionally deficient B cells that are unable to undergo immunoglobulin class-switching and antibody production. 1 SCID-X1 is caused by mutations in the gene encoding the common γ chain ( γc) of several interleukin receptors. In April 2000, a team at Hôpital Necker-Enfants Malades in Paris reported the successful use of gene therapy to treat two infants with the X-linked form of severe combined immunodeficiency (SCID-X1). Only partial immunological reconstitution was achieved, most likely because of the relatively low dose of gene-corrected CD34+ cells re-infused, although viral infection during the early phase of T-cell reconstitution and the infant’s NK+ phenotype may also have exerted an effect. He went on to receive a bone marrow transplant from a matched unrelated donor 26 months after gene therapy.Ĭonclusions: This is the first occasion that gene therapy has been used to treat a genetic disease in Australia. The infant failed to thrive and developed weakness, hypertonia and hyperreflexia in the legs, possibly the result of immune dysregulation. However, T-cell levels did not reach the reference range, and immune reconstitution remained incomplete. Within 2 weeks of the appearance of T cells, there was a distinct clinical improvement, with early weight gain and clearance of rotavirus from the gut. Results: T cells were observed in peripheral blood 75 days after treatment, and levels increased rapidly to 0.46 × 10 9 CD3+ cells/L at 5 months. Gene-modified cells (equivalent to 1.3 × 10 6 CD34+/ γc+ cells/kg) were returned to the infant via a central line. Cells were then genetically modified by exposure to a retrovirus vector encoding human γc (the common γ chain of several interleukin receptors mutations affecting the γc gene cause SCID-X1). Procedure: CD34+ haemopoietic progenitor cells were isolated from harvested bone marrow and cultured with cytokines to stimulate cellular replication. Patient: A 9-month-old male infant with confirmed SCID-X1 (including complete absence of T cells) with an NK+ phenotype (a less common variant of SCID-X1), and no HLA-identical sibling donor available for conventional bone marrow transplantation. Follow-up to March 2005 (36 months) is available. Objective: To report the outcome of gene therapy in an infant with X-linked severe combined immunodeficiency (SCID-X1), which typically causes a lack of T and natural killer (NK) cells.ĭesign and setting: Ex-vivo culture and gene transfer procedures were performed at The Children’s Hospital at Westmead, Sydney, NSW, in March 2002. Statistics, epidemiology and research design.


 0 kommentar(er)
0 kommentar(er)
